HELP CLEAR THE WAY WITH BIMZELX NAVIGATE®

Rapid
Access
Access: Eligible, commercially insured patients who experience an insurance coverage delay or denial can obtain their first dose of BIMZELX through BIMZELX Navigate Bridge.*
$5 or $15
Affordability: Eligible, commercially insured patients may pay just $15 per dose of BIMZELX for up to 2 years while their insurance coverage is pending.* Eligible, commercially insured patients may be eligible to receive BIMZELX for as little a $5 per dose.†
1 Point
of Contact
Support: Our Nurse Navigators serve as your patient’s single point of contact for answering questions they may have, from insurance coverage to injection training to financial assistance and much more.‡

BIMZELX Navigate helps make the treatment journey smooth from the start for your patients.
*For eligible commercially insured patients only. Eligible patients who have a delay or denial of coverage pay as little as $15 per dose of BIMZELX for up to two years or until the patient’s commercial insurance plan approves coverage, whichever comes first. Please see full eligibility and terms at BIMZELX.com/patient-support/navigate-benefits.
†Eligible commercially insured patients may pay $5 per dose. View complete eligibility requirements and terms by clicking on the button below or by visiting BIMZELX.com/patient-support/navigate-benefits.
‡Nurse Navigators do not provide medical advice and will refer patients to their healthcare professional for treatment-related questions.
Full Terms and Conditions for BIMZELX Navigate® Bridge
BIMZELX Navigate® Bridge (the “Program”) provides BIMZELX® (bimekizumab-bkzx) to eligible patients for $15 per dose for up to two (2) years or until the patient’s commercial insurance plan approves coverage for the drug, whichever comes first. Eligible patients must be 18 years of age or older with commercial insurance and a valid prescription consistent with FDA-approved product labeling. For initial enrollment into the Program, the patient must be experiencing a delay in, or have been denied, coverage for BIMZELX by their commercial insurance plan. To maintain eligibility in the Program, the following are required: (1) a prior authorization request has been submitted and/or coverage remains unavailable for the patient; and (2) if the prior authorization is denied by the payer, the prescriber must submit an appeal within the first sixty (60) days of the prior authorization denial and a prior authorization must be submitted every six (6) months thereafter or documentation as may otherwise be required by the payer. Program is not available (1) to patients whose prescriptions are reimbursed, in whole or in part, under Medicare (including Medicare Part D), Medicaid, or any other federal- or state-funded health care programs (including any state prescription drug assistance programs and the Government Health Insurance Plan available in Puerto Rico), (2) where a patient’s insurance covers the drug, (3) to uninsured or cash-paying patients, or (4) where otherwise prohibited by law. Product shall be dispensed pursuant to Program rules and federal and state laws. Patients may be asked to re-verify insurance coverage status during participation in the Program. No purchase necessary. Program is not health insurance, nor is participation a guarantee of insurance coverage. Limitations may apply. This Program cannot be combined with any other savings, free trial, or similar offer for the specified prescription. The patient, or healthcare provider on the patient’s behalf, must not submit any claim for reimbursement for product provided under this Program to any third-party payer. UCB, Inc. reserves the right to end or amend this Program without notice.
Full Terms and Conditions for BIMZELX Navigate® Savings
BIMZELX Navigate® Savings (the “Program”) provides BIMZELX® (bimekizumab-bkzx) to eligible patients with commercial insurance coverage for as little as $5 per dose. Eligible patients must be 18 years of age or older with commercial insurance coverage with a valid prescription consistent with FDA approved product labeling. The Program is not available for (1) for prescriptions that are reimbursed, in whole or in part, under Medicare (including Medicare Part D), Medicaid, or any other federal- or state-funded healthcare programs (including any state prescription drug assistance programs and the Government Health Insurance Plan available in Puerto Rico), (2) where a patient’s commercial insurance plan reimburses for the entire cost of the drug, (3) for uninsured or cash paying patients, or (4) where otherwise prohibited by law. Product shall be dispensed pursuant to Program rules and federal and state laws. The value of the Program is exclusively for the benefit of patients and is intended to be credited in full towards patient out-of-pocket obligations and maximums, including applicable co-payments, coinsurance and deductibles. Patient may not seek reimbursement for the value of this Program from other parties, including third-party payers (i.e., any health insurance program or plan, or public payers like Medicare, Medicaid, Medigap, TRICARE, VA, and DoD). Patient is responsible for complying with any applicable limitations and requirements of their health plan related to the use of the Program. This Program cannot be combined with any other savings, free trial, or similar offer for the specified prescription. UCB, Inc. reserves the right to amend or end this Program at any time without notice. Subject to the prior sentence, this Program expires at 11:59 p.m. on December 31. Patients that meet the above requirements may re-enroll in the Program each year.
PATIENT SAVINGS/SUPPORT PROGRAM OVERVIEW
Get more information on the BIMZELX Navigate program.
BC CORNER
The Biologic Coordinator (BC) training portal provides helpful information such as detailed instructions on how to use the BIMZELX autoinjector and prefilled syringe.
FOR SPECIALITY PHARMACY USE: BIMZELX NAVIGATE CAN HELP PATIENTS PAY AS LITTLE AS $5
We have two options for your eligible, commercially insured patients:
• Pay as little as $5 once insurance coverage is approved
• Pay just $15 per dose for up to 2 years while insurance coverage is pending
For eligible patients who may have difficulty paying for medication, we can help find resources to assist with cost. Visit UCB Savings to sign up patients for copay assistance, and visit the HCP BIMZELX Navigate portal for Specialty Pharmacy resources.
COMPREHENSIVE SUPPORT FOR STARTING PATIENTS ON BIMZELX
ENROLLMENT AND PRIOR AUTHORIZATION/APPEALS MATERIALS

Get patients started on BIMZELX and enrolled in BIMZELX Navigate.

Explore tips on how to properly fill out a new patient enrollment form and see a sample completed form.

Review checklists and samples of the Prior Authorization and Letter of Medical Necessity forms.

Use this template to draft a Letter of Medical Necessity to accompany a Prior Authorization and/or Appeal.

Use this letter template to draft a Letter of Appeal following a claims denial.
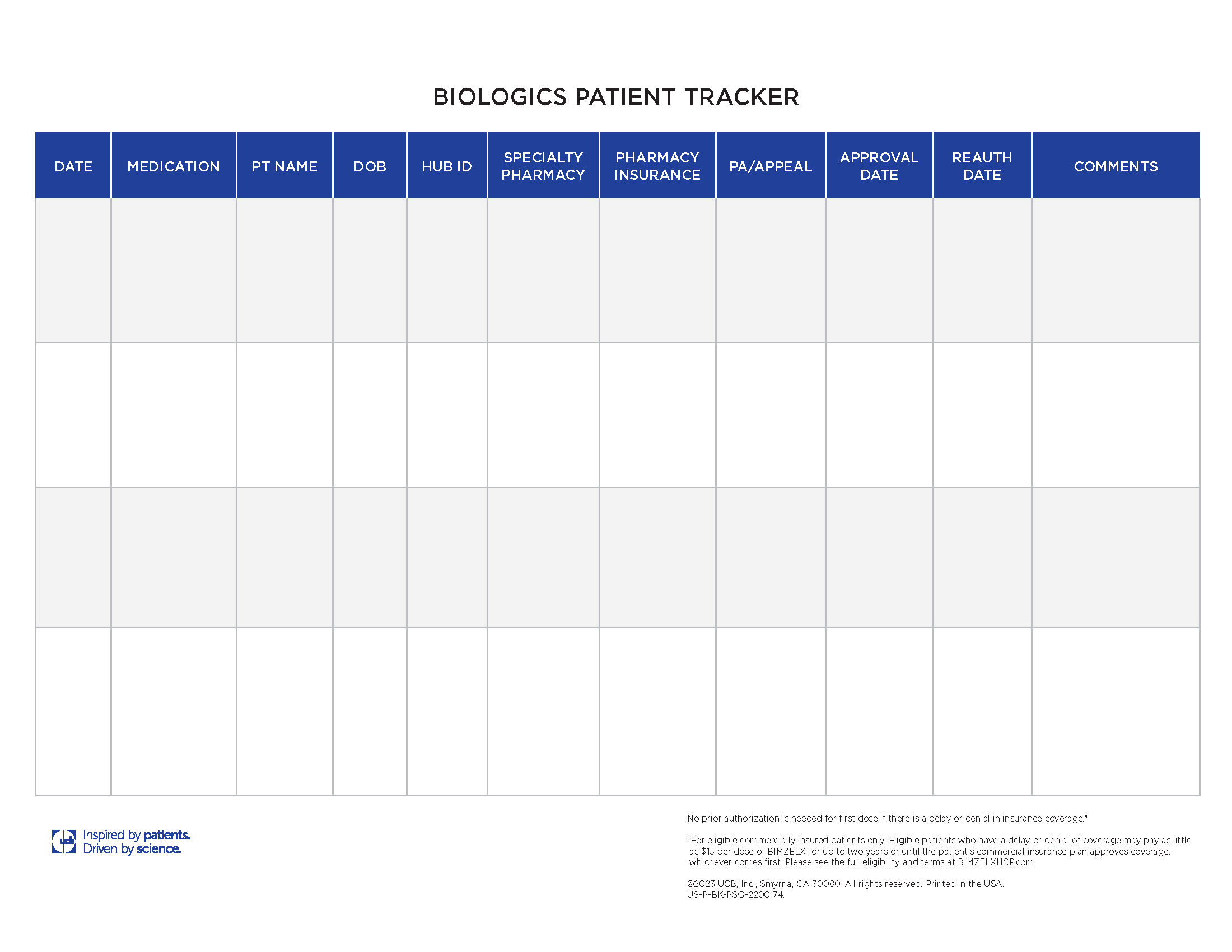
Track patient milestones like prior authorization, appeals status, and approval dates.

Use this template to draft a letter to request that BIMZELX be considered a preferred tier medication.
PHARMACY AND PATIENT MATERIALS
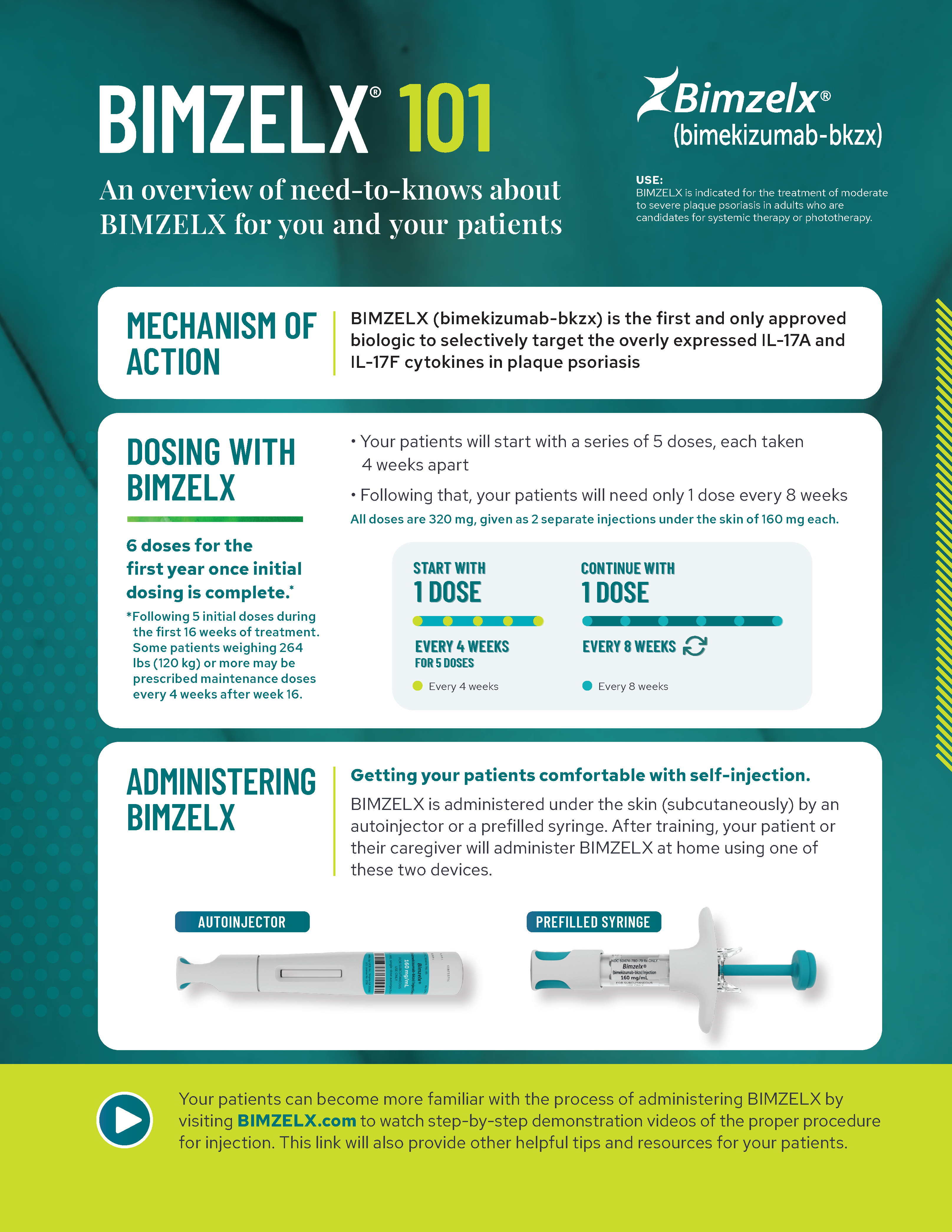
Review key information about BIMZELX, including clinical data, safety profile, and dosing options.

Review instructions for using the BIMZELX autoinjector and prefilled syringe.

Review a list of specialty pharmacy contacts and services.

GET BIMZELX EDUCATIONAL RESOURCES FROM LEADING DERMATOLOGY EXPERTS
WANT MORE INFORMATION ABOUT BIMZELX?
