PASI 100 CONSISTENTLY DEMONSTRATED ACROSS HEAD-TO-HEAD CLINICAL TRIALS2-4
Results like this make dermatologists like us stand up and take notice.
Watch how BIMZELX is reaching higher in psoriasis treatment by targeting COMPLETE CLEARANCE (PASI 100) in head-to-head clinical trials against COSENTYX® (secukinumab), HUMIRA® (adalimumab), and STELARA® (ustekinumab). Then explore the data demonstrating why patients can expect RAPID results and MAINTAIN longer-term clearance with BIMZELX.
Treatment Response Time
Learn how you can give many of your patients the opportunity to achieve skin clearance that can last.
Head-to-Head
Hear how BIMZELX performed in clinical trials against three of the most prescribed biologics.
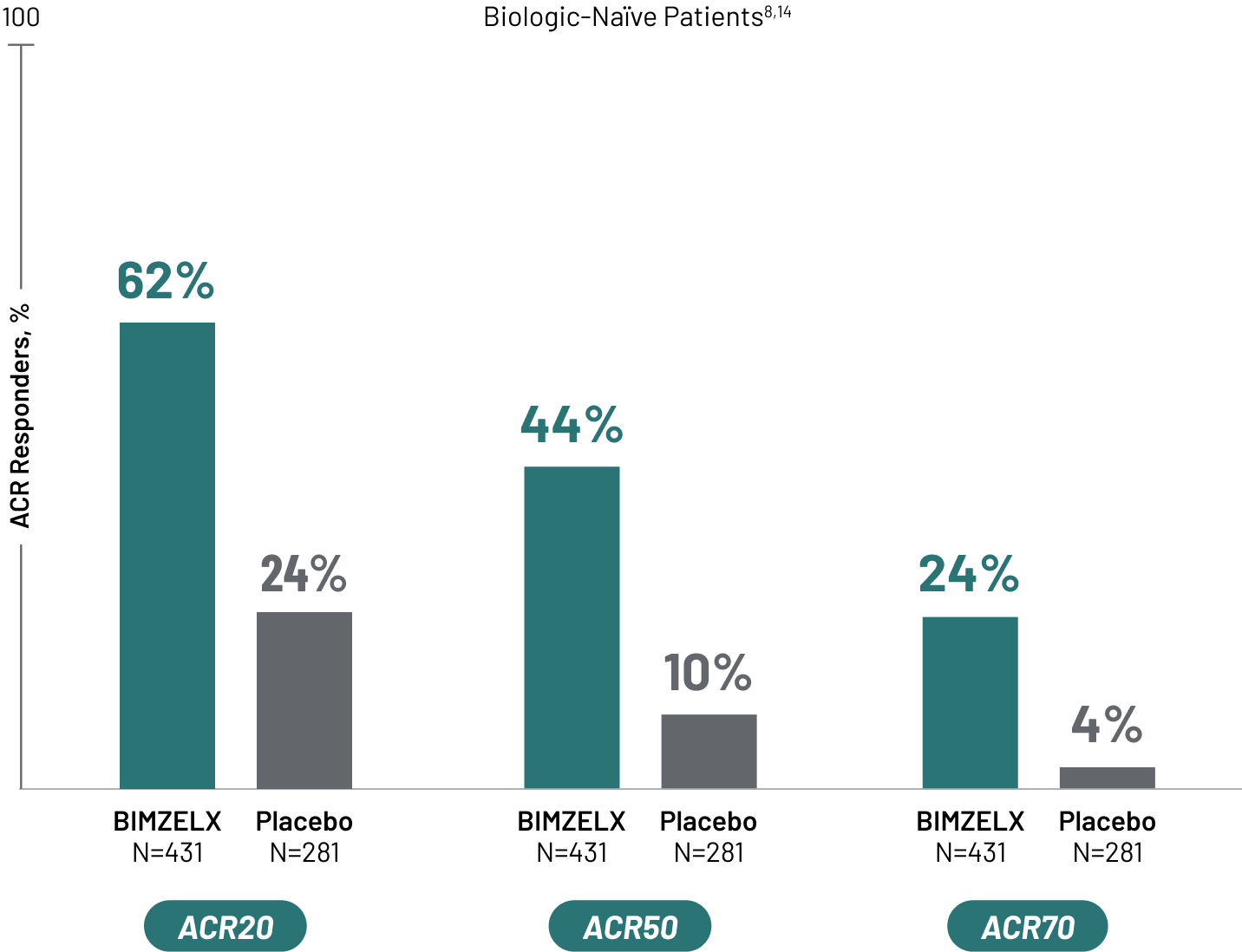
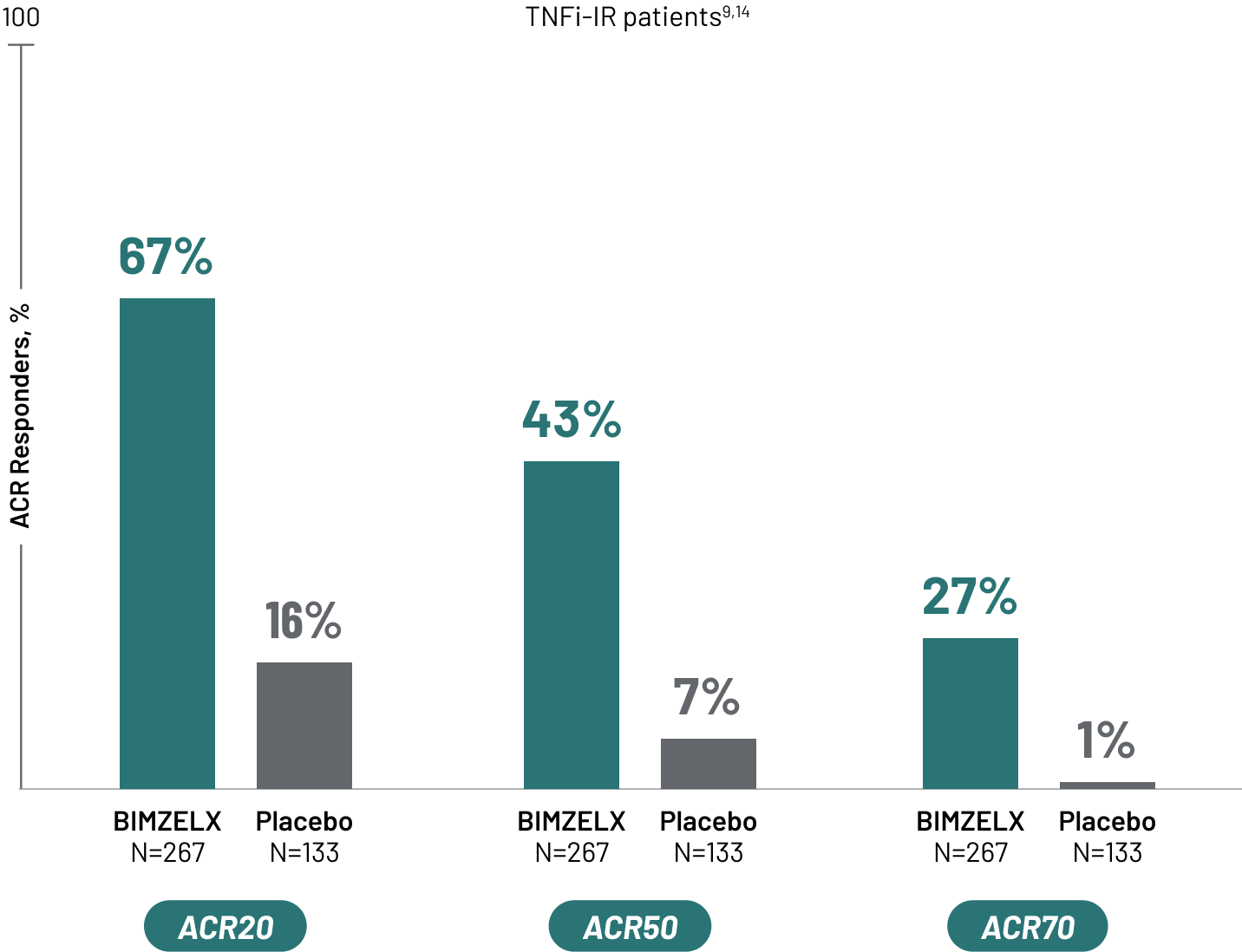
BIMZELX REACHES FOR AND ACHIEVES A HIGHER TREATMENT TARGET IN PSORIATIC ARTHRITIS CLINICAL TRIALS
American College of Rheumatology 20% Response Criteria (ACR20) is a composite measure defined as an improvement of 20% in the number of tender and swollen joints and a 20% improvement in three of the following criteria: physician’s global assessment, acute phase reactant, and three patient-reported outcomes. Those outcomes include HAQ-DI, patient pain assessment, and patient global assessment.7
Thus far, ACR20 has been the most commonly used response index and the primary outcome for most clinical trials in psoriatic arthritis, but ACR50 and ACR70 are the same instruments with improvement levels defined as 50% and 70%, respectively.7 Bimekizumab is the first biologic to assess ACR50 as early as Week 16 as a primary outcome in psoriatic arthritis clinical trials.1,8,9
SIGNIFICANT JOINT IMPROVEMENT ACHIEVED AT WEEK 16
All patients in the BIMZELX group received 160 mg Q4W.
TNFi-IR=tumor necrosis factor inhibitor-inadequate responder. Q4W=every 4 weeks.
BIMZELX HALTED THE ADVANCEMENT OF STRUCTURAL JOINT DAMAGE in psoriatic arthritis trials AS EARLY AS WEEK 16
Change From Baseline in vdHmTSS
in BE OPTIMAL8
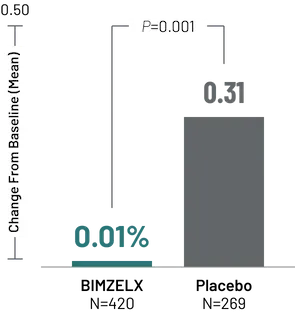
All patients in the BIMZELX group received 160 mg Q4W. All patients in the placebo group were switched to BIMZELX 160 mg Q4W at 16 weeks.
Change from baseline in vdHmTSS at Week 16 was a ranked secondary endpoint in BE OPTIMAL.8 Radiographic outcomes were not evaluated in BE COMPLETE.9
vdHmTSS=van der Heijde-Modified Total Sharp Score.
What does the vdHmTSS measure?11
Psoriatic arthritis produces persistent inflammation of the joints that can lead to irreversible, structural joint damage over time and bring about diminished physical function and a lower quality of life in patients with psoriatic arthritis.
The vdHmTSS, adapted from the original Sharp Method for rheumatoid arthritis, evaluates structural joint damage and emphasizes symptoms unique to psoriatic arthritis, such as distal interphalangeal (DIP) joint involvement, and prioritizes joint erosion in its scoring.
Joint Space Narrowing Score + Erosion Score = vdHmTSS Score
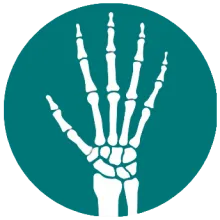
28 joints are assessed
in the hands
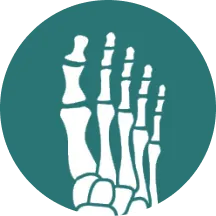
12 joints are assessed
in the feet
BIMZELX is a major breakthrough. It has results for psoriatic arthritis that exceed what we have seen for all the other biologics. It has results for psoriasis where, for the first time, the majority of patients at the primary endpoint achieved PASI 100. And this is one of the first times we can say a significant number of psoriatic arthritis patients achieved ACR50 and ACR70, instead of just ACR20.12
THE BIMZELX DIFFERENCE IS IN THE MOA*
BIMZELX is the first and only approved biologic to inhibit
*The clinical relevance of the mechanism of action is unknown.
Across different patient populations with different levels of inflammatory skin disease, the amazing thing was the consistency we saw with BIMZELX‑highlighting that IL-17A and IL-17F are really at the heart of this skin disorder.
Targeting IL-17A and IL-17F
Discover the significance of IL-17F in psoriasis pathogenesis.
BIMZELX: The Discovery
Learn the story behind BIMZELX from Dr. Stevan Shaw, VP, Head of Immunology Research for UCB.
A CONSISTENT SAFETY PROFILE WITH 4 YEARS OF DATA6
The chronic nature of psoriasis requires persistent management, making long-term safety assessment of treatment a cornerstone for clinical decision-making. BIMZELX demonstrated a consistent safety profile over 4 years with low incidences of adverse events across the pooled analysis of short-term and long-term safety data.6
Robust Safety Data Across 5 Phase 3/3b Clinical Trials6
| SHORT TERM (PHASE 3)* Weeks 0-16 % (n) |
LONGER TERM (PHASE 3/3B)† Year 4 EAIR/100 PY (95% CI) |
||
|---|---|---|---|
| BIMZELX 320 MG (n=989) | PLACEBO (n=169) | BIMZELX ALL DOSES (n=2,186) | |
|
Candida infections |
9.1% (90) |
0% |
10.4 (9.5, 11.3) |
| Oral candidiasis |
7.6% (75) |
0% |
8.9 (8.1, 9.7) |
| Injection site reactions |
2.7% (27) |
1.2% (2) |
1.7 (1.4, 2.0) |
| ALT or AST elevations |
|
|
|
| >3x ULN
|
1.3% (13) |
1.2% (2) |
1.9 (1.6, 2.3) |
| >5x ULN‡ |
0.3% (3) |
0% |
0.5 (0.4, 0.7) |
|
Malignancies (inc. NMSC) |
0.4% (4) |
0.6% (1) |
0.9 (0.6, 1.1) |
|
Serious infections |
0.3% (3) |
0% |
1.3 (1.0, 1.6) |
|
Adjudicated MACE |
0.1% (1) |
0% |
0.6 (0.4, 0.8) |
|
Adjudicated inflammatory bowel disease§ |
0.1% (1) |
0% |
0.2 (0.1, 0.3) |
|
Adjudicated suicidal ideation and behavior |
0% |
0% |
0.1 (0.1, 0.2) |
|
Serious hypersensitivity reactionsll |
0% |
0% |
0.1 (0.0, 0.2) |
| * Pooled short-term data from Weeks 0-16 of 3 Phase 3 trials (BE SURE, BE VIVID, and BE READY).6 † Longer-term data from 5 Phase 3/3b trials (Phase 3: BE SURE, BE VIVID, BE READY and their OLE BE BRIGHT; Phase 3b: BE RADIANT). BE RADIANT ran for 3 years; therefore, the total pooled exposure only includes BE RADIANT data to Year 3 (cut-off May 6, 2022) in addition to BE BRIGHT data to Year 4 (cut-off Nov. 14, 2022).6 ‡ Patients with elevations >5x ULN were a subset of patients with elevations >3x ULN. § Includes any TEAE adjudicated as definite or probable IBD.6 Long-term data included in the BIMZELX Prescribing Information includes seven cases of new onset IBD (including ulcerative colitis, Crohn’s disease, and IBD-undetermined) in subjects exposed to BIMZELX (0.12 per 100 PY).1 II No anaphylactic reactions associated with BIMZELX were reported.6 ALT=alanine aminotransferase. AST=aspartate aminotransferase. CI=confidence interval. EAIR=exposure-adjusted incidence rate. IBD=inflammatory bowel disease. MACE=major adverse cardiovascular events. NMSC=non-melanoma skin cancer. PY=patient-year. ULN=upper limit of normal. |
|||
Safety Q&A
Were patients with a known history of inflammatory bowel disease, or IBD, excluded from the BIMZELX trials?
- BIMZELX [prescribing information]. Smyrna, GA: UCB, Inc.
- Gordon KB, et al. Lancet. 2021;397(10273):475-486.
- Reich K, et al. Lancet. 2021;397(10273):487-498.
- Warren RB, et al. N Engl J Med. 2021;385(2):130-141.
What were the recurrence rates of candidiasis in the
BIMZELX trials?
Data on file. UCB, Inc., Smyrna, GA.
How often do I need to monitor for elevated liver transaminases?
BIMZELX [prescribing information]. Smyrna, GA: UCB, Inc.
Can you tell me more about the data behind the Warning and Precaution around suicidal ideation and behavior in the BIMZELX label?
- BIMZELX [prescribing information]. Smyrna, GA: UCB, Inc.
- Posner K, et al. Am J Psychiatry. 2011;168(12):1266-1277.
- Data on file. UCB, Inc., Smyrna, GA.
Investigator ExperienceS
Hear as two investigators share their clinical experiences with BIMZELX in the Phase 3 clinical trials.
Investigator Experience–Jamie Weisman, MD
Clinical trial investigator Jamie Weisman, MD, of Atlanta Medical Dermatology Specialists, Inc., discusses her experience with BIMZELX.
Investigator Experience–Jeffrey Weinberg, MD
Clinical trial investigator Jeffrey Weinberg, MD, of Infinity Dermatology NYC and Mt. Sinai, discusses his experience with BIMZELX.
References
1. BIMZELX [prescribing information]. Smyrna, GA: UCB, Inc. 2. Reich K, et al. N Engl J Med. 2021;385(2):142-152. 3. Reich K, et al. Lancet. 2021;397(10273):487-498. 4. Warren RB, et al. N Engl J Med. 2021;385(2):130-141. 5. Gordon KB, et al. Lancet. 2021;397(10273):475-486. 6. Data on file. UCB, Inc., Smyrna, GA.
08/2024 US-BK-2400881

EXPLORE RAPID, COMPLETE, AND MAINTAINED CLEARANCE FROM THE VERY FIRST DOSE1-5*
*From the very first dose as measured at Week 4;
results were not immediate.
WANT MORE INFORMATION ABOUT BIMZELX?
INDICATIONS
BIMZELX is indicated for the treatment of adults with active psoriatic arthritis, active non-radiographic axial spondyloarthritis with objective signs of inflammation, active ankylosing spondylitis, moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy, and moderate-to-severe hidradenitis suppurativa.
IMPORTANT SAFETY INFORMATION
Suicidal Ideation and Behavior
BIMZELX may increase the risk of suicidal ideation and behavior (SI/B). A causal association between treatment with BIMZELX and increased risk of SI/B has not been definitively established. Prescribers should weigh the potential risks and benefits before using BIMZELX in patients with a history of severe depression or SI/B. Advise monitoring for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct to promptly seek medical attention, refer to a mental health professional as appropriate, and re-evaluate the risks and benefits of continuing treatment.
Infections
BIMZELX may increase the risk of infections, including serious infections. Do not initiate treatment with BIMZELX in patients with any clinically important active infection until the infection resolves or is adequately treated. In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing BIMZELX. Instruct patients to seek medical advice if signs or symptoms suggestive of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and do not administer BIMZELX until the infection resolves.
Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX. Avoid the use of BIMZELX in patients with active TB infection. Initiate treatment of latent TB prior to administering BIMZELX. Consider anti-TB therapy prior to initiation of BIMZELX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Closely monitor patients for signs and symptoms of active TB during and after treatment.
Liver Biochemical Abnormalities
Elevated serum transaminases were reported in clinical trials with BIMZELX. Test liver enzymes, alkaline phosphatase, and bilirubin at baseline, periodically during treatment with BIMZELX, and according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt BIMZELX until a diagnosis of liver injury is excluded. Permanently discontinue use of BIMZELX in patients with causally associated combined elevations of transaminases and bilirubin. Avoid use of BIMZELX in patients with acute liver disease or cirrhosis.
Inflammatory Bowel Disease
Cases of inflammatory bowel disease (IBD) have been reported in patients treated with IL-17 inhibitors, including BIMZELX. Avoid use of BIMZELX in patients with active IBD. During BIMZELX treatment, monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs.
Immunizations
Prior to initiating therapy with BIMZELX, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with BIMZELX.
MOST COMMON ADVERSE REACTIONS
Most common (≥1%) adverse reactions in plaque psoriasis and hidradenitis suppurativa include upper respiratory tract infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other candida infections, and fatigue.
Most common (≥2%) adverse reactions in psoriatic arthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infections.
Most common (≥2%) adverse reactions in non-radiographic axial spondyloarthritis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, transaminase increase, and urinary tract infections.
Most common (≥2%) adverse reactions in ankylosing spondylitis include upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection site pain, rash, and vulvovaginal mycotic infection.
Please see the full Prescribing Information.
Most common (≥2%) adverse reactions in PsA, nr-axSpA, and AS include upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infections. Other most common (≥2%) adverse reactions specific to each indication include: urinary tract infections (PsA); cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, transaminase increase, and urinary tract infections (nr-axSpA); injection site pain, rash, and vulvovaginal mycotic infections (AS).